Thermal analysis
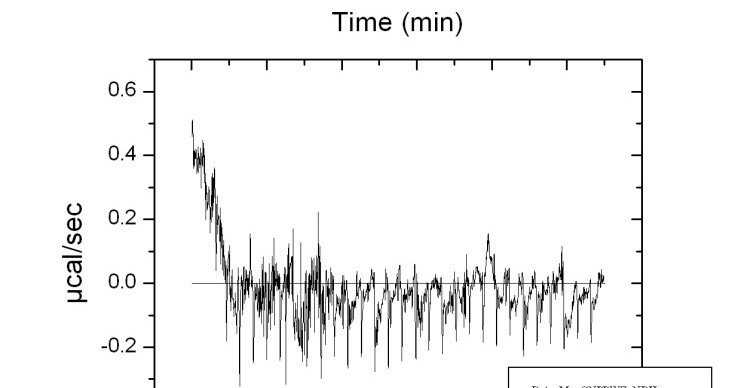
IC Isothermal Calorimetry
Isothermal Titration Calorimetry (ITC) is a technique that measures the heat that is either released or absorbed during a biomolecular binding event, providing information binding affinity and thermodynamics: binding constants (KD), reaction stoichiometry (n), enthalpy (∆H) and entropy (∆S).
TC/D Thermal conductivity/diffusivity
TEMPORARILY UNAVAILABLE
The laser flash method allows to measure materials thermal diffusivity with high accuracy (typically below 3%) and in a wide range of temperatures.
TGA Thermogravimetric Analysis (TGA)
The thermal analyses are the set of techniques in which a physical property of a substance is measured as a function of temperature (or time) while the sample is subjected to a controlled temperature program (heating, cooling, isotherm).
DSC Differential Scanning Calorimetry (DSC)
Differential Scanning Calorimetry (DSC) measures the difference in the amount of heat required to increase the temperature of a sample and reference as a function of temperature and therefore considers its energetic variation.
STG-DSC-DTA Simultaneous TG-DSC-DTA
In the Thermogravimetric Analysis (TGA) the mass of a sample is measured as a function of temperature or time, while the Differential Scanning Calorimetry (DSC) measures the difference in the amount of heat required to increase the temperature of a sample and reference as a function of temperature.
HTM HT-microscopy
The heating microscope allows to “visually” follow what occurs to the specimens subjected to heat treatment. In this way, it is possible to achieve information about: melting of the material (softening temperature, hemisphere temperature and sphere temperature).