Cell culture facilities
Nano to Micro/Macro (in vitro assays and cell analysis)
The cell culture facility comprises of two fully equipped cell and stem-cell culture laboratories, a biochemical and molecular biology laboratory, a chemistry lab for sample preparation and imaging instruments (eg. SEM, epifluorescence and Confocal microscope and non-linear microscope). In the Ultrafast Laser Micro and Nano Processing Laboratory (ULMNP) of IESL-FORTH, the objective is to investigate the biocompatibility of laser-engineered biomimetic 3D scaffolds fabricated on hard metallic and soft polymeric materials, exhibiting different micro/nano topographies and surface energies and to understand the cell-biomaterial interaction under static and dynamic (under flow) culture conditions, in vitro. In the Tissue Engineering – Regenerative Medicine and Immunoengineering Lab (TERMIM Lab) of IESL-FORTH, the research is focused on understanding the physicochemical mechanisms that take place at the interface between cells and biomaterials in micro / nano scale and the examination of the potential medical and/or clinical applications of optimized artificial tissue scaffolds.
In general, the cell culture facility has the equipment, protocols and assays for the following research activities:
- In-vitro cytotoxicity testing of metallic and polymeric surfaces
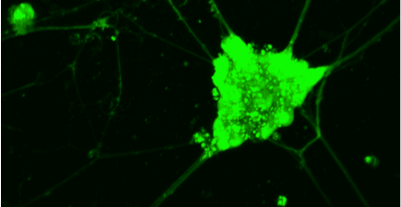
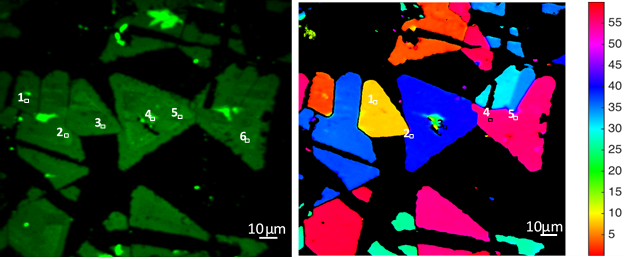
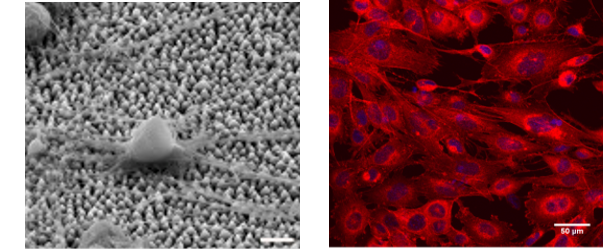
- In-vitro cytocompatibility studies of various cells (eg. mouse neuronal cell lines, mouse neural stem cells, mouse mesenchymal stem cells, fibroblasts, human cancer cell lines) on the above biomaterial architectures/patterns as shown in Figure 1.
- Proteomic and biomolecular profiles of cell lines adhered on bio-based surfaces
- Study of the effect of flow-induced shear stress on cells adhered on biomaterial surfaces
- Ca+2 imaging, electrical activity and live imaging of neural stem cells and neuronal cell lines interacting with bio-based materials via a non-linear microscopy system
- Antibacterial testing of biomimetic surfaces and structured materials
- Βiophysical studies of model lipid membranes
Instruments datasheets