The best way of anchoring single noble metal atoms
Single atom catalysts (SACs) are catalytically active materials featuring single noble metal atoms anchored at the surface of metal oxide substrates. In this configuration, each single noble metal atom represents a catalytic site yielding ultimate noble metal efficiency. Presently SACs face challenges related to the low density of active sites and poor thermal stability. Both factors are controlled by the availability of the specific anchoring sites, typically defects, and the coordination environment of the noble metal cations.
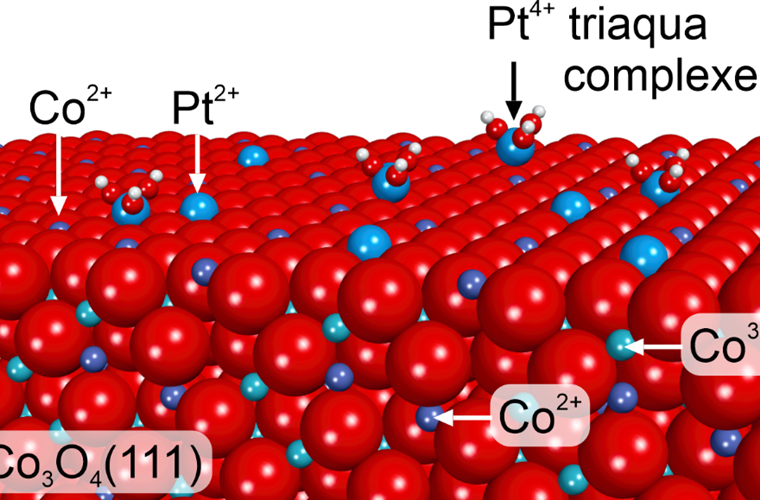
Nanostructuring of the oxide substrates may increase the densities of anchoring sites, but this is not sufficient to achieve a high coverage of atomically dispersed noble metals. Therefore, new strategies are needed to fabricate SACs with a high density of single-atom sites, ideally on defect-free supports. In this respect, the best way of anchoring single noble metal atoms is to employ the phenomenon of cation place exchange at the surface of cation-terminated oxide substrate. In this process, the noble metal atoms occupy the position of the host cations triggering their migration into the bulk. We found that the corresponding phenomenon occurs upon deposition of Pt atoms onto the Co2+-terminated surface of the Co3O4(111) thin films at room temperature. Here, Pt atoms substitute Co2+ cations assuming the oxidation state Pt2+.

Pt species formed on Co3O4(111) observed by means of STM (top) and assigned based on computed STM patterns (bottom). Adapted from L. Fusek et al., J. Mater. Chem. A 2024, 12 (6), 3258-3264 licensed under CC BY 3.0 DEED
“NFFA-Europe has presented us with a valuable opportunity to carry out our research within a network of scientific facilities equipped with cutting-edge techniques and methodologies”, concludes Lykhach.
Ideally, it should be possible to substitute all surface Co cations with Pt species yielding SAC with utmost density of active sites. In the presence of co-adsorbed molecular water, single noble metal atoms can be stabilized in the form of triaqua complexes with Pt atoms in Pt4+ state. The corresponding species have been observed by scanning tunnelling spectroscopy (STM) and assigned with the help of density function theory (DFT). Annealing above 600 K in ultrahigh vacuum triggers sub-surface diffusion of Pt species followed by its place exchange with sub-surface Co3+ cations.
“We were able to obtain new insights into the charge distribution and charge transfer between Pt species and Co cations upon surface and sub-surface cation place exchange by combining synchrotron radiation photoelectron spectroscopy (SRPES) and DFT methods”, says Dr. Yaroslava Lykhach. The obtained knowledge provides a basis for the rational design of SAC with high density of atomically dispersed noble metal species.
Fusek L., Camellone M. F., Ronovsky M., Kastenmeier M., Skála T., Samal P. K., Tsud N., Mehl S., Skvára J., Dolák T., Uvarov V., Setvin M., Johánek V., Fabris S., Brummel O., Libuda J., Myslivecek J., Piccinin S., Lykhach Y. Atomistic picture of electronic metal support interaction and the role of water. J. Mater. Chem. A 2024, 12 (6), 3258-3264
Theory and Simulation
ATOMS AND MOLECULES IN MOTION
STRUCTURAL AND GROUND-STATE ELECTRONIC PROPERTIES
at CNR-IOM

Yaroslava Lykhach
Dr. Yaroslava Lykhach is a researcher at the Chair of Interface Research and Catalysis, Department of Chemistry and Pharmacy, Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany. The expertise of Dr. Yaroslava Lykhach is the investigation of chemical reactions at the surfaces and interfaces of well-defined model catalysts by means of spectroscopic techniques including synchrotron radiation photoelectron spectroscopy (SRPES) and resonant photoemission spectroscopy (RPES) with a special emphasis on redox interactions and charge transfer. Dr. Yaroslava Lykhach manages the research activities that involve synchrotron radiation. She coordinates a team of researchers performing scheduled experiments at synchrotrons Elettra (Italy), DESY (Germany), ESRF (France), MAX IV (Sweden). She is the author and co-author of more than 70 publications in peer-reviewed journals with more than 3500 citations and h-index 25.

Olaf Brummel
Dr. Olaf Brummel is a group leader at the Chair of Interface Research and Catalysis at the Friedrich-Alexander-Universität Erlangen-Nürnberg in Germany. His expertise is centered around model electrocatalysis in energy conversion with a special focus on reaction mechanisms and kinetics at electrified solid/liquid interfaces. His group employs model systems with different levels of complexity including simple single crystalline surfaces, real electrocatalysts, and complex, well-defined nanostructured model electrocatalysts prepared by surface science methods. His group uses a variety of electrochemical in-situ methods for mechanistic studies with a special emphasis on electrochemical infrared spectroscopy. By studying the same reactions in ultra-high vacuum and electrochemical environment, he aims at bridging the gap between fundamental electrochemical surface science and applied electrochemistry.

