Near-ambient pressure X-ray photoelectron spectroscopy enables in-situ observation of dynamic covalent chemistry in two-dimensional frameworks.
|
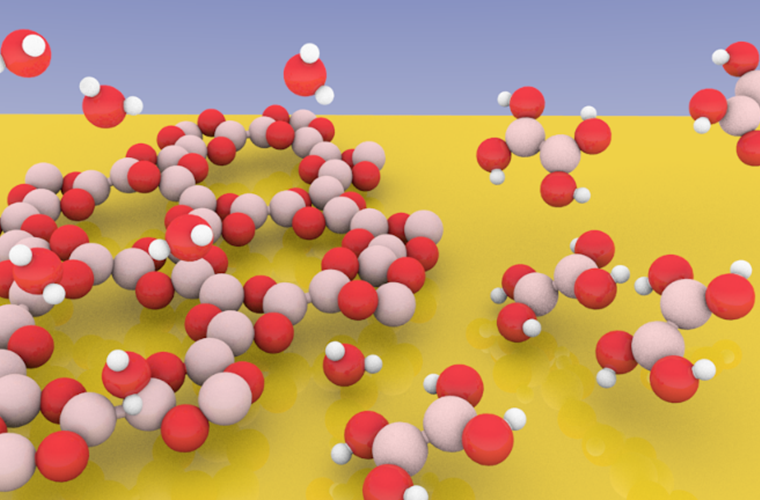
Covalent organic frameworks (COFs) are reticular materials formed by the linkage of organic building blocks to create periodic structures with predefined topologies. This peculiar characteristic enables the construction of tailor-made systems for applications in catalysis and optoelectronics. Two-dimensional (2D) COFs are structurally like graphene sheets within graphite, exhibiting strong intralayer covalent bonds and weak interlayer coupling. 2D-COFs can be fabricated through on-surface synthesis, which makes use of atomically flat surfaces as templates to confine condensation reactions in two dimensions. However, although on-surface synthesis in ultra-high vacuum (UHV) enables the growth of atomically thin COFs, the resulting degree of crystallinity is typically low. This issue is caused by the irreversibility of covalent bond formation, which limits the synthesis of ordered structures. “As the water partial pressure is a key parameter to determine the chemical equilibrium of the system, systematic studies are required to identify the conditions which favor the formation of crystalline structures”, says Paul Leidinger. “The formation of crystalline structures requires a mechanism for error correction, where covalent bonds can be broken and reformed,” says Laerte Patera. Such an approach, called dynamic covalent chemistry, is based on the Le Chatelier‘s principle, stating that the addition of the reaction by-products (water in this case) allows the system to repair itself, providing a mechanism for error correction which facilitates the formation of the most stable crystalline structure. “As the water partial pressure is a key parameter to determine the chemical equilibrium of the system, systematic studies are required to identify the conditions which favor the formation of crystalline structures”, says Paul Leidinger. As water pressures in the millibar range are typically required for dynamic covalent chemistry, conventional X-ray photoelectron spectroscopy (XPS) cannot be used, as this approach is limited to high vacuum conditions. Therefore, near-ambient pressure X-ray photoelectron spectroscopy (NAP-XPS) experiments have been performed at the NAPP end station of the CIRCE beamline at the ALBA synchrotron. Such an instrument allows probing surface reactions of up to 20 mbar of gas pressure. As a result, by tuning the sample temperature and the water pressure in the experimental chamber, controlled formation or dissolution of the framework has been achieved, as evidenced by NAP-XPS. “We have shown that reversible bond formation in 2D-COFs can be probed by current NAP-XPS analyzers, allowing the extension of such approach to a large variety of organic networks,” says Laerte Patera This study was performed for a boroxine network, being a prototypical two-dimensional polymer. In this case, under typical conditions for 2D-COF growth (about 120 °C), a pressure of 0.5 mbar is needed to enable covalent bond breaking. Complementary scanning tunneling microscopy (STM) experiments were performed at the VT-STM instrument at CNR-IOM to characterize the process at the atomic scale. There the dissolution of the framework has been carried out in a high-pressure cell connected to the microscope chamber. These results represent the first in-situ observation of dynamic covalent chemistry in 2D-COFs, being the stepstone for the growth of crystalline polymeric networks. “We have shown that reversible bond formation in 2D-COFs can be probed by current NAP-XPS analyzers, allowing the extension of such approach to a large variety of organic networks. Furthermore, it opens to the possibility to precisely determine relevant parameters, as the kinetic barriers for reversible reaction and the Gibbs free energy, ruling dynamic covalent bond formation,” says Laerte Patera
|
|
P. Leidinger, M. Panighel, V. P. Dieste, I. J. Villar-Garcia, P. Vezzoni, F. Haag, J. V. Barth, F. Allegretti, S. Günther and L. L. Patera, Nanoscale, 15, 1068-1075 (2023). |
NAPP end station of the CIRCE beamline at ALBA synchrotron. The differentially pumped electron energy analyzer enables near-ambient pressure X-ray Photoelectron Spectroscopy (NAP-XPS) experiments from ultra-high vacuum (UHV) conditions up to 20 mbar.
VT-STM at CNR-IOM. This facility allows high-resolution in-situ scanning tunneling microscopy in a wide range of temperatures and pressures. A high-pressure cell connected to the UHV chamber enables the characterization of samples exposed to high pressure (up to 100 mbar) of gas.

Laerte Patera
Laerte Patera is Assistant Professor at the University of Innsbruck (Austria). He received his PhD in Nanotechnology from the University of Trieste (Italy) in 2016 and worked as a postdoctoral researcher at the University of Regensburg (Germany) from 2016 to 2019. In 2020 he started his independent career at the Technical University of Munich (Germany). He was awarded the Gustav Hertz Prize of the German Physical Society (2019) and an ERC Starting Grant (2022). His research is focused on the synthesis and imaging of two-dimensional covalent organic frameworks for energy conversion.

